Partnered with

Hi HAIFers,
This week marks the launch of Health AI Deep Dives, a new newsletter by HAIF focused on in-depth analysis of individual AI point solutions. The first Deep Dive is here, breaking down Doximity, who have kindly sponsored today’s newsletter. The response has been strong, with 2,300+ subscribers joining organically within four days.
Sponsored by: Doximity
DoxGPT is a HIPAA-compliant AI assistant designed for U.S. healthcare professionals. It streamlines clinical and administrative tasks by answering clinical questions with cited, evidence-based references - including integrated drug information -generating chart notes and documentation, and creating patient education materials.
DoxGPT integrates seamlessly with Doximity tools and ensures robust data security. Free for verified clinicians, DoxGPT reduces documentation burden and improves access to up-to-date medical knowledge, so you can focus more on patient care.

Your shortcut for this week:
Read on for the foresight of the week and the full breakdown of what shaped healthcare AI 👇
Signal of the week
Shadow AI Is Becoming Visible at Scale
The Physicians AI Report shows clinicians using 50+ unvetted tools. [Link]. This is the same week that FDA device lists and EU fast-track guidance expand in parallel. Innovation is happening bottom -up, governance top-down - creating a widening gap between how AI is used and how it’s officially managed.
Foresight:
Health systems will move from blocking AI to absorbing it. Expect formal “approved AI stacks,” and clearer guardrails that legitimise clinician-led adoption while managing risk. Shadow AI won’t disappear - it will be institutionalised.
Solutions and Launches
PathAI (US): AIM‑MASH AI Assist became the first FDA‑qualified AI pathology tool for MASH trials, validated on 1,400+ biopsies and proven more reproducible than manual reads for biopsy scoring. [Link]
Flow Neuroscience (US/Global): Received FDA PMA approval for its headset to treat major depressive disorder. Crucially approved as a first-line monotherapy, validating at-home neuromodulation as a primary treatment option, not just a last resort. [Link]
Sleep.ai (US): Launched Sleep Sense, an API/SDK that turns smartphones into passive, privacy‑preserving sleep sensors. Using multimodal AI inference trained on nearly 1B hours of sleep data, it targets the 85% of adults not tracking sleep, enabling population‑level measurement without wearables or audio recording. [Link]
Medical Care Technologies (US): Completed its first AI consumer nutrition app, entering iOS and Google Play testing with plans to deploy in 100+ countries and 30+ languages while continuing development of its AI melanoma detection tool. [Link]
Holly Health (UK): A digital health coaching startup; integrated its automated behavioural support tool into Patient.info, one of the UK’s largest health information portals. [Link]
Respiree (Singapore): Secured HSA approval for 1Bio AI‑Acute, the country’s first AI tool to predict patient deterioration. It analyses bedside vitals and EMR data to forecast ICU transfers with greater precision than traditional early warning scores, helping reduce alarm fatigue. [Link]

Governance, Policy, and Ethics
European Commission (EU): Finalised guidance for the "breakthrough technologies" pathway under the MDR/IVDR. A pilot launching in early 2026 will test faster regulatory approvals for high-impact innovations while maintaining safety standards. [Link]
FDA (US): Updated its official lists of digital health technologies, now cataloguing 1,357 AI-enabled devices and 234 sensor-based tools. A vital resource for distinguishing regulated medical devices from general wellness tools. [Link]
Physicians AI Report (US): Revealed widespread "Shadow AI" usage, with doctors using 50+ unvetted tools. It highlights a governance gap: clinicians are bypassing institutional blockers to solve workflow pain points. [Link]
US Executive Order (US): President Trump issued a directive to create a national AI policy framework, pre-empting conflicting state laws and establishing an AI Litigation Task Force. [Link]
Research and AI Advancements
Ophthalmology Triage Pilot Study: Tested Vision-Language Models (VLMs) combined with Chain-of-Thought (CoT) reasoning. [Link]
Eindhoven University & Amsterdam UMC (Netherlands): Released GastroNet-5M, a massive open dataset of 4.8 million endoscopy images [Link]
Promises and challenges of multimodal AI in healthcare, noting applications in sepsis and cardiology, and barriers such as data integration, fairness, and safety. Strategies like federated learning are proposed to support responsible adoption and equitable outcomes. [Link]

Medical-Reasoning-SFT-GPT-OSS-120B just got released, an open dataset of 200k step-by-step clinical reasoning chats. Designed to train models on how to think- prioritising depth and differential diagnosis over short answers. [Link]
NetraMark published a paper showing its explainable AI platform (NetraAI) improved patient selection for depression trials by ~30% over standard methods, achieving 95% accuracy in identifying responders. [Link]

Feature article warns that rising use of AI chatbots as companions may both ease loneliness and risk deepening social isolation, highlighting evidence of short‑term mental health benefits but concerns about dependency and reduced human connection. [Link]
UW Health (US): NEJM AI trial showed ambient AI notetaking reduced clinician burnout, cut documentation time by about 30 minutes daily, and improved note accuracy; now used by 800 clinicians across Wisconsin and Illinois. [Link]
Brainomix (UK): study across 70+ hospitals found the Brainomix 360 Stroke AI platform cut transfer times by 64 minutes and doubled thrombectomy rates. [Link]
Meta-analysis found mpMRI-based AI outperformed radiologists in predicting prostate cancer extension. [Link]
Experts call for independent, openly reported head‑to‑head validation of AI medical devices to ensure safe, transparent deployment in national screening programmes. [Link]
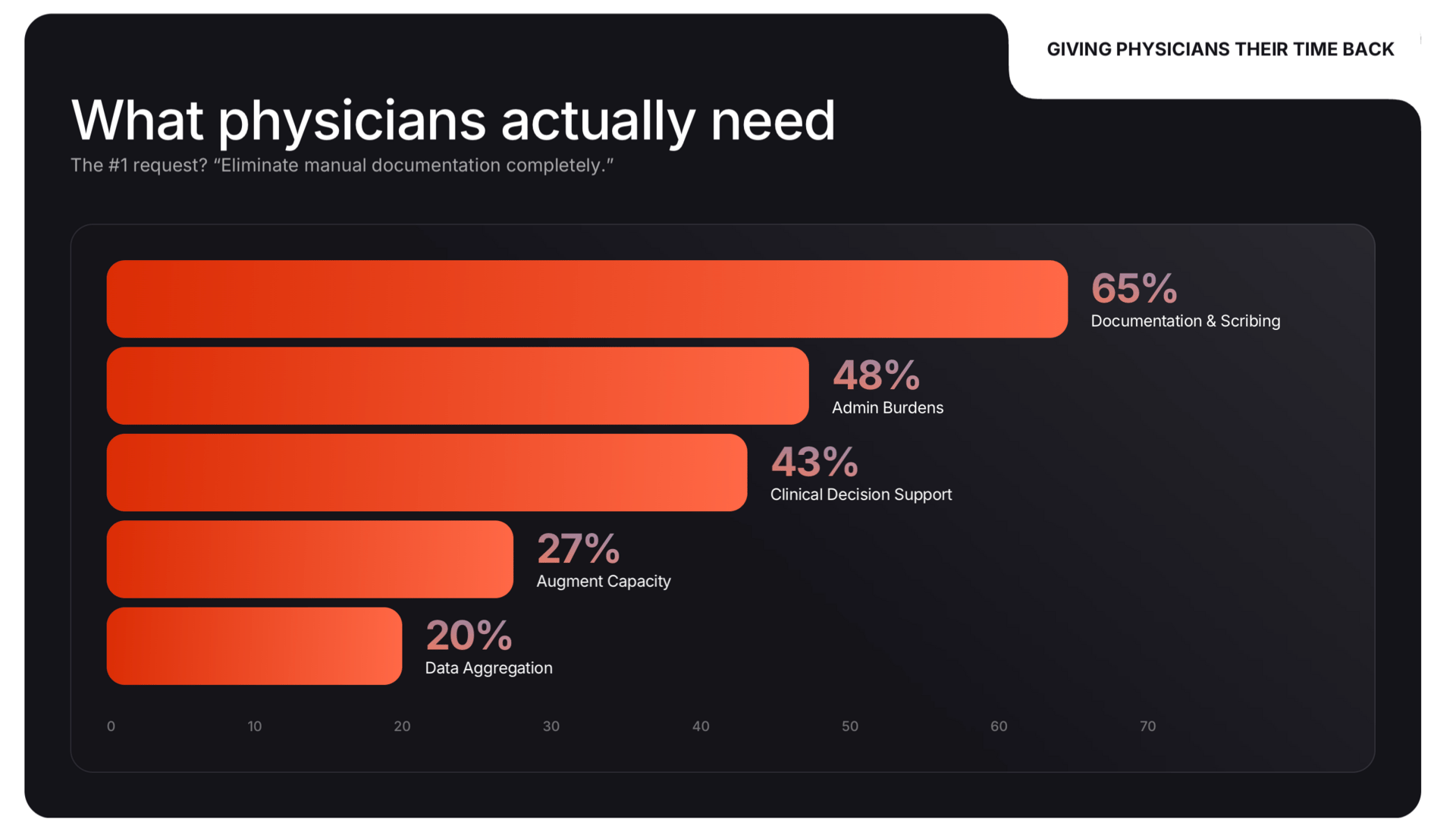
Sword Health has released MindEval, an open-source framework that tests LLMs in simulated, 40+ turn therapy sessions against strict clinical criteria. The findings show models like GPT-5 and Claude 4.5 scored below 4 out of 6 on clinical reliability, struggling specifically with severe symptoms and maintaining context over long conversations. [Link]
Tandem Health (Europe): Published results from a massive pilot with Capio, generating 375k notes. The data shows a 29% reduction in documentation time and a 30% drop in administrative stress. [Link]
Partnerships & Adoption
Thatch & MyOme (US): Partnered to launch the first genomics solution in Thatch’s benefits marketplace. MyOme’s Proactive Health platform offers whole‑genome screening with single‑gene risk insights, pharmacogenomics, and polygenic risk scores. [Link]

Accurx (UK): Integrated its ambient voice tool directly with Oracle (Cerner) EPR. [Link]
NHS Confederation & Limbic (UK): Partnered to explore safe adoption of AI in mental health services, focusing on governance, safety, and practical guidance. [Link]
Qure.ai & Dutch Hospitals (Netherlands): Catharina Ziekenhuis and UMC Utrecht adopted Qure.ai’s software to automate lung nodule detection, aiming to improve early lung cancer diagnosis. [Link]
WHOOP (APAC): Partnered with Standard Chartered to offer health coaching and wearables to banking clients. [Link]
Bets on the Next Health System
Investments:
Chai Discovery (US): Raised $130 million to advance its molecular "computer-aided design suite," reaching a $1.3 billion valuation. [Link]
OpenEvidence (US): Raising $250M at a $12B valuation which is its second major raise since October, doubling its valuation in two months. [Link]
Medra (US): Raised $52 million to scale its "Physical AI Scientist" platform, which uses robotics and vision to automate wet lab experimentation. [Link]
Ritten (US): Raised $35M Series B led by Five Elms Capital to expand its AI-powered behavioural health platform, streamlining care and operations for mental health and addiction providers. [Link]
M&A:
Philips (Netherlands): Agreed to acquire SpectraWAVE to integrate FDA-cleared AI intravascular imaging into its image-guided therapy portfolio. [Link]
That’s a wrap for Edition #10 of Health AI Foresight.
My goal with this newsletter is simple - to connect the present, the emerging, and the future of healthcare AI.
See you next week.
- Dr. Aboufandi
📩 Something stand out? Or have a suggestion? Hit reply - I read every message.
